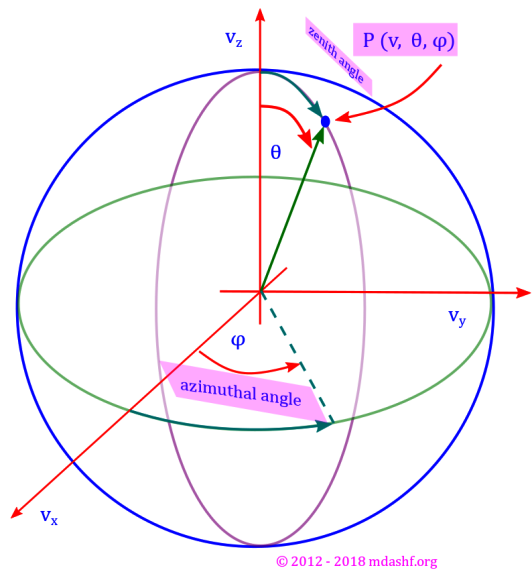
Tag: spherical polar coordinate
-
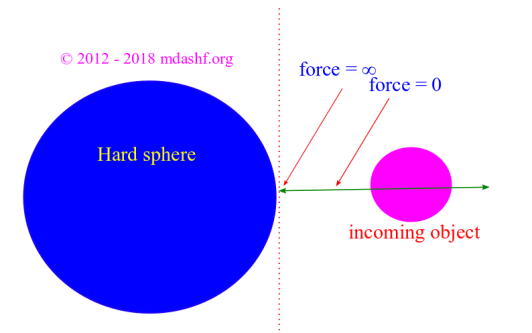
Maxwell Boltzmann distribution for a classical ideal gas
i. We assume a dilute gas which is enclosed by a thermally insulated container on all sides. Dilute gas in a thermally insulated container: Dilute means concentration of gas molecules is low. Insulated implies there is no reasonable flow of heat energy across the walls of the container. ii. Each molecule is assumed to be a…